Auto-generate site tools and push protocol logic into EDC, CTMS, eTMF, and payments—so trials start faster and run cleaner.

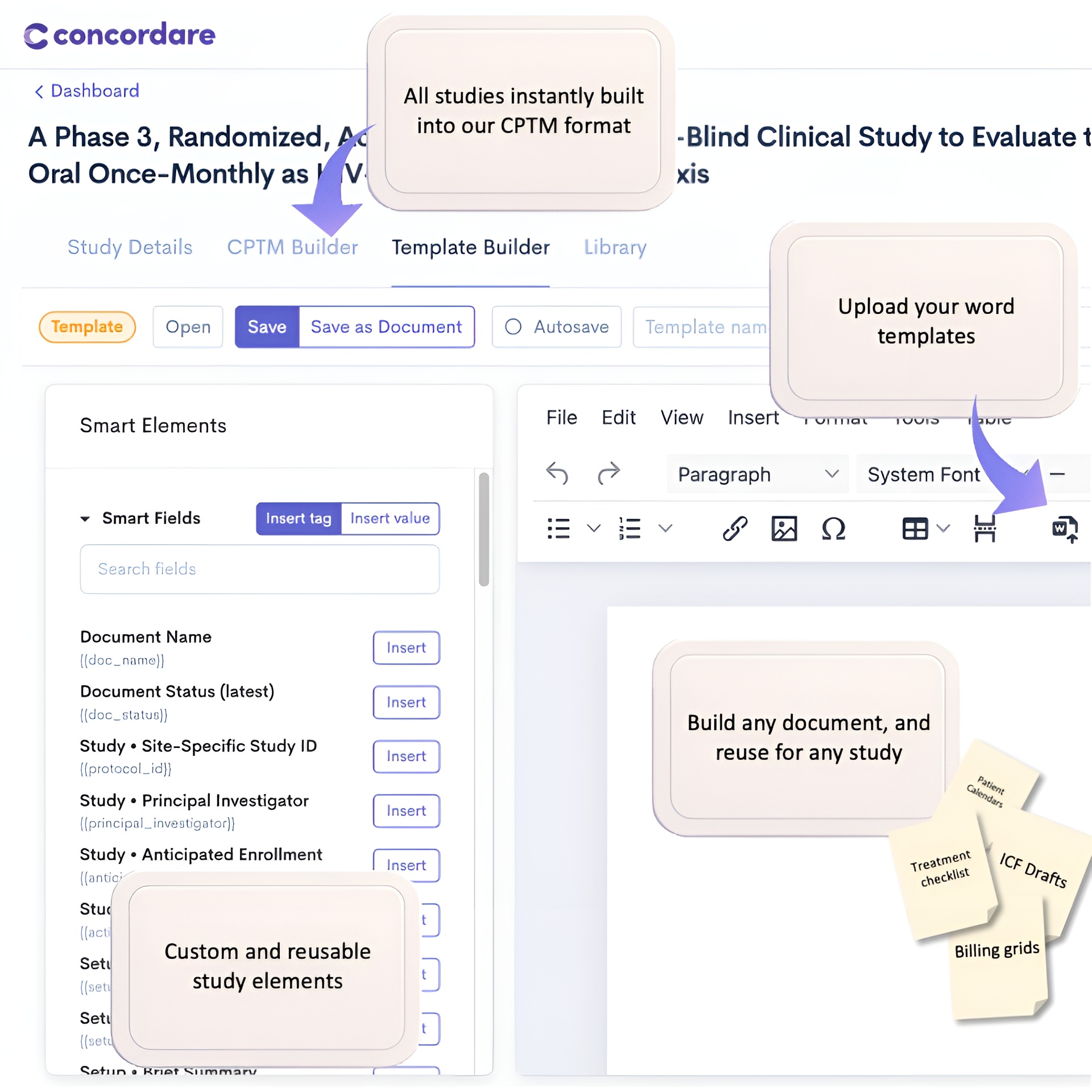
The web app that (1) converts any protocol into CPTM using AI and (2) instantly generates high-quality operational tools: patient calendars, billing grids, consent templates, treatment checklists, and study trackers, contracts, and much more.
Upload your protocol (draft or final, any format)
Our AI-to-CPTM conversion engine creates the CPTM digital protocol for a universal source of truth
Use the Concordare Suite to instantly generate custom operational tools and documents
Connect the CPTM (export, API, ingestions, partner connectors) so your CPTM drives downstream systems
Share & version once—everyone stays in sync (sites, sponsors, CROs)
Designed for API and file-based exchange; roadmap includes native connectors to leading CTMS/EDC/eTMF and EHR partners. CPTM naming aligns to CDISC to simplify mapping.
Reads CPTM and instantly generates high-quality operational tools — patient calendars, billing grids, treatment checklists, consent templates, and trackers — so sites can use the protocol immediately.
Derive visit schedule, windows, and data-collection requirements from CPTM so forms and edit checks align with the protocol out-of-the-box.
Populate milestones, monitoring visit plans, task lists, and site activation checklists directly from the protocol backbone.
Generate and version protocol-driven artifacts (ICF drafts, calendars, checklists) and keep them synchronized.
Translate visit/procedure logic into payment schedules and invoice rules. (no more hand-built billing grids)
Concordare prioritizes effective clinical trial site operations. Patients, sponsors, and site staff benefit when sites are able to rapidly operate a clinical trial and reinforce the ability to meet a study’s specific requirement.
Works for any study at any site—not locked to a single vendor ecosystem
Built from clinical ops reality; zero heavy implementation to get value quickly
CPTM makes protocol logic consistent and machine-readable (CDISC-aligned)
From single-site IITs to global Phase III; share the same CPTM across teams and systems
Concordare is hosted on a secure, cloud-native infrastructure with industry-standard encryption, access controls, and multi-factor authentication. We do not store Protected Health Information (PHI), HIPAA compliance is not required for our current operations.
We are building our platform with 21 CFR Part 11–ready architecture — versioned protocol files, audit trails, and role-based access. This ensures our customers can rely on Concordare for traceability, data integrity, and audit readiness.




