
CPTM is the digital backbone of every trial. It standardizes protocol logic into a format all stakeholders can trust, update, and connect to their systems.
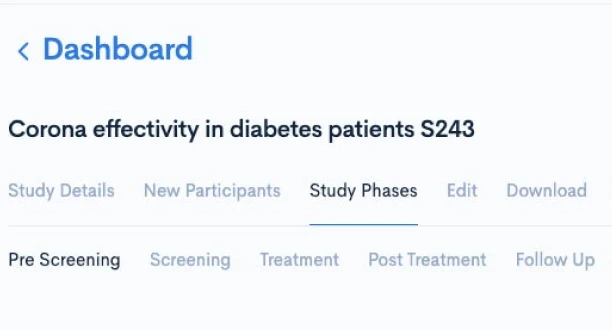
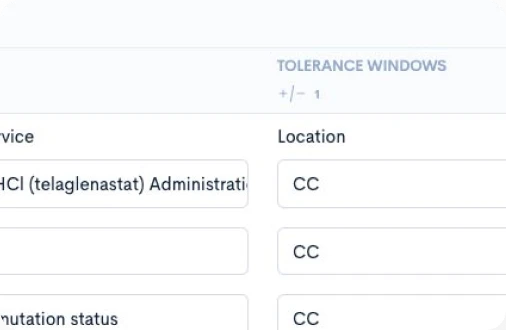
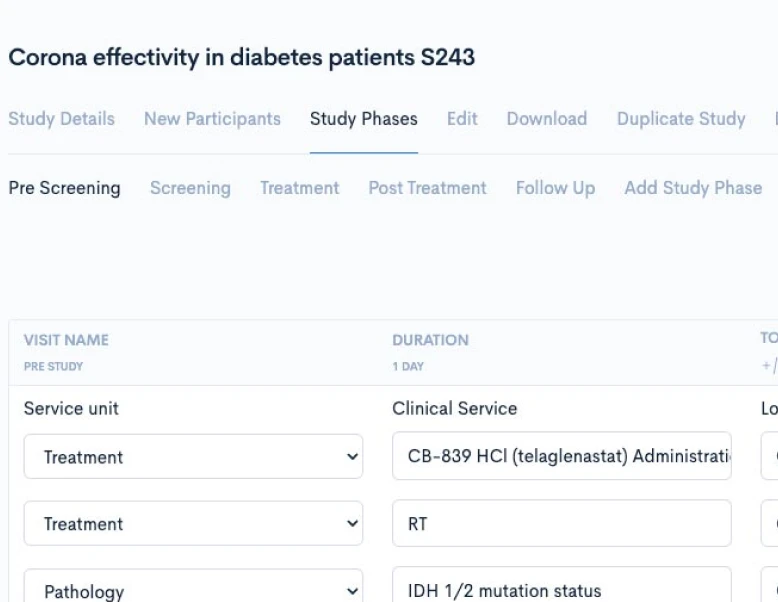

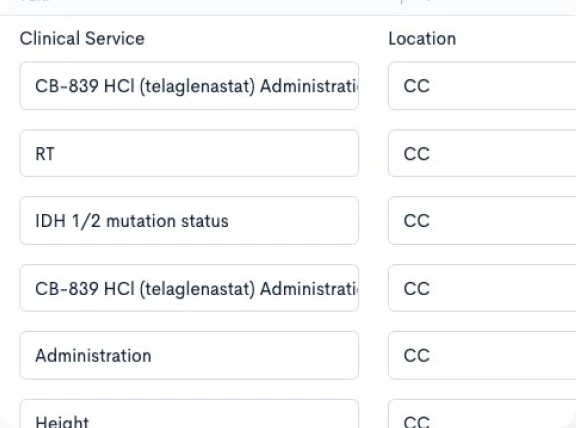
Creating a CPTM doesn’t require new authoring software or complex training. Concordare’s AI-powered converter takes any existing protocol — draft or final, in Word or PDF — and transforms it into a machine-readable CPTM file.
Auto-build visit schedules, forms, and edit checks.
Populate milestones, monitoring visits, and site tasks.
Sync informed consents, calendars, and trackers.
Generate billing grids and payment schedules automatically.
Enable proactive deviation detection and audit-ready traceability.
CPTM is built for interoperability and traceability. While Concordare doesn’t store PHI, the system uses encryption and audit trails. The architecture is 21 CFR Part 11–ready to support compliance as customers require it.